Wireless, batteryless implantable sensors could improve brain control of prosthetics, avoiding wires that go through the skull. (UC Berkeley video by Roxanne Makasdjian and Stephen McNally)
Because these batteryless sensors could also be used to stimulate nerves and muscles, the technology also opens the door to «electroceuticals» to treat disorders such as epilepsy or to stimulate the immune system or tamp down inflammation.
The
«I think the
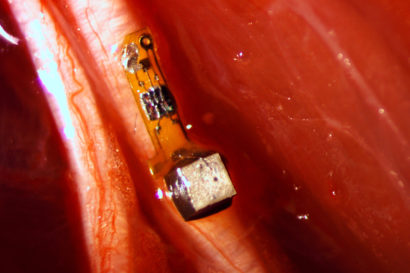
The sensor, 3 millimeters long and 1×1 millimeters in cross section, attached to a nerve fiber in a rat. Once implanted, the batteryless sensor is powered and the data read out by ultrasound. (Ryan Neely photo)
The sensors, which the researchers have already shrunk to a 1 millimeter cube — about the size of a large grain of sand — contain a piezoelectric crystal that converts ultrasound vibrations from outside the body into electricity to power a tiny,
Motes sprinkled thoughout the body
In their experiment, the UC Berkeley team powered up the passive sensors every 100 microseconds with six
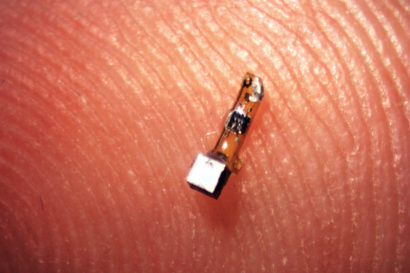
The sensor mote contains a piezoelectric crystal (silver cube) plus a simple electronic circuit that responds to the voltage across two electrodes to alter the backscatter from ultrasound pulses produced by a transducer outside the body. The voltage across the electrodes can be determined by analyzing the ultrasound backscatter. (Ryan Neely photo)
«The original goal of the neural dust project was to imagine the next generation of
In a paper published online in 2013, the researchers estimated that they could shrink the sensors down to a cube 50 microns on a side — about 2 thousandths of an inch, or half the width of a human hair. At that size, the motes could nestle up to just a few nerve axons and continually record their electrical activity.
«The beauty is that now, the sensors are small enough to have a good application in the peripheral nervous system, for bladder control or appetite suppression, for example," Carmena said. «The technology is not really there yet to get to the
The team is working now to miniaturize the device further, find more biocompatible materials and improve the surface transceiver that sends and receives the ultrasounds, ideally using

Diagram showing the components of the sensor. The entire device is covered in a biocompatible gel.
«The vision is to implant these neural dust motes anywhere in the body, and have a patch over the implanted site send ultrasonic waves to wake up and receive necessary information from the motes for the desired therapy you want," said Dongjin Seo, a graduate student in electrical engineering and computer sciences. «Eventually you would use multiple implants and one patch that would ping each implant individually, or all simultaneously.»
Ultrasound vs radio
Maharbiz and Carmena conceived of the idea of neural dust about five years ago, but attempts to power an implantable device and read out the data using radio waves were disappointing. Radio attenuates very quickly with distance in tissue, so communicating with devices deep in the body would be difficult without using potentially damaging
Marharbiz hit on the idea of ultrasound, and in 2013 published a paper with Carmena, Seo and their colleagues describing how such a system might work. «Our first study demonstrated that the fundamental physics of ultrasound allowed for very, very small implants that could record and communicate neural data," said Maharbiz. He and his students have now created that system.
«Ultrasound is much more efficient when you are targeting devices that are on the millimeter scale or smaller and that are embedded deep in the body," Seo said. «You can get a lot of power into it and a lot more efficient transfer of energy and communication when using ultrasound as opposed to electromagnetic waves, which has been the
«Now that you have a reliable, minimally invasive neural pickup in your body, the technology could become the driver for a whole gamut of applications, things that today don’t even exist," Carmena said.
Other


