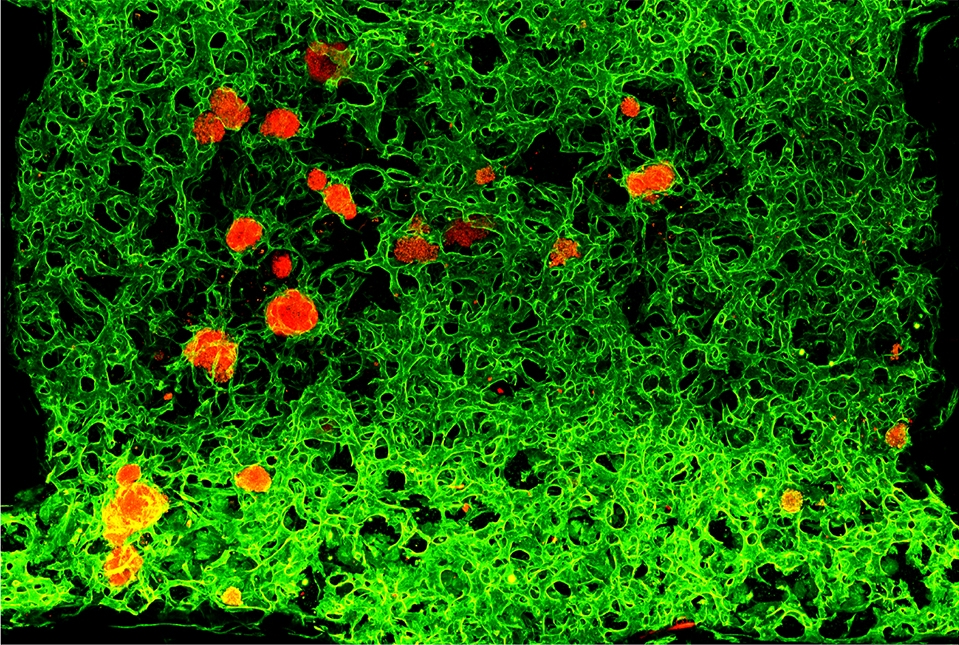
Transplantable human pancreatic islets (red) with integrated network of blood vessels (green) in laboratory dish. Image courtesy of Nature.
The scientists, whose study was published Sept. 9 in Nature, found that a key protein could rejuvenate adult human endothelial cells - the building blocks of blood vessels – returning them to a malleable state in which they readily grow and conform to surrounding tissues.“This advance allows us to generate a tissue-specific network of functional blood vessels to nourish and support a variety of model organs, or organoids, as well as the development of transplantable human pancreatic islets, which can be used for research studies and potentially for organ repair,” said senior author Dr. Shahin Rafii, the director of the Ansary Stem Cell Institute at Weill Cornell Medicine (WCM). “We can also now decipher how cancerous blood vessels acquire their abnormal features, permitting identification of new druggable targets for tumors.”
Blood vessel networks for organ regeneration
Shortages of donor organs and deficiencies in animal models of human disease pose hurdles in developing therapeutics and repairing or replacing injured organs. Transplanted organs may fail because of poor blood supply. Additionally, blood vessels in each organ and tumor are different and success in regenerating damaged organs or targeting cancerous ones requires an understanding of how to customize blood vessels.Current “organ-on-chip” models in which miniaturized tissues are lined by a single layer of blood vessels have shortcomings, as these technologies do not permit blood vessels to interact with and adjust to other cell types and their environment. “There is still no protocol approved by U.S. regulators to regenerate organs, largely due to the inability to get blood vessels to grow within artificial organs that can be connected to the host blood supply when transplanted,” said co-senior author Dr. Sina Rabbany, adjunct associate professor of bioengineering in medicine at WCM and dean of the DeMatteis School of Engineering and Applied Science at Hofstra University.
The new study is based on a discovery by first author Dr. Brisa Palikuqi, a former postdoctoral associate in Dr. Rafii’s lab, that a protein called ETV2, can profoundly change the properties of adult human blood vessels cells grown in culture. ETV2 is a “pioneer transcription factor,” or one that can reprogram cells by switching on or off a broad set of genes.
“Adult endothelial cells don’t know how to make new blood vessels from scratch,” said Dr. Palikuqi, who is now a postdoctoral scholar at University of California, San Francisco. “Our idea was to use ETV2 to reset vascular endothelial cells (abbreviated as R-VECs) to a fetal state in which they can adaptively form new vessels based on signals from the surrounding tissue. Thus, they are re-educated to perform specialized vascular functions. We also identified a mixture of three natural tissue-molding ‘matrix’ proteins that helped R-VECs to form blood vessels in devices that carry fluids and blood. With this 3-D platform, which we call “Organ-On-VascularNet,” we can use R-VECs to build tissue-specific blood vessels that may help regenerate organs.”
The researchers used specialized imaging methods to show that R-VECs in this natural matrix could self-assemble into an extensive functional vessel network capable of carrying human blood in a lab dish, a feat that the investigators believe has not been accomplished before. “Three-dimensional visualization of human blood vessels being assembled and sculpted in real time has been challenging,” said Dr. Ryan Schreiner, who is an assistant professor of cell physiology research in ophthalmology and director of the Visual Function Core at WCM. “The capacity of R-VECs to self-organize and form blood vessels, which then carry human blood, is instructive to watch and may help us to identify pathways choreographing this marvel of nature.”
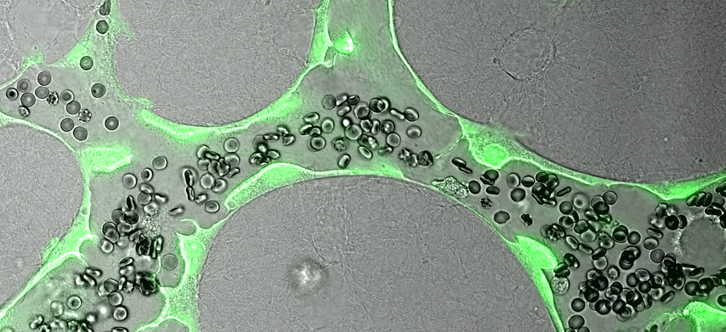
Engineered human vascular capillaries shown transporting blood in lab dishes designed for screening candidate therapies. Image courtesy of Dr. Ryan Schreiner and Dr. Ge Li.
Customized blood vessels for disease modeling and therapeutics
The investigators showed that the vessels readily connected to the existing vasculature of mice and remained viable for several months. They also demonstrated that the R-VEC vessels supported the growth of lab-grown healthy or cancerous organoids. “One can imagine the opportunities R-VECs create for regenerative medicine and cancer targeting,” said co-author Dr. Duc-Huy T. Nguyen, a postdoctoral associate in medicine at WCM. “We can now generate in the lab vascularized tissue models that closely mimic human diseases, overcoming the need to employ experimental animals.”To demonstrate the versatility of their technology, the team showed that in laboratory devices, R-VECs can vascularize and support the function of human islets—clusters of insulin-producing cells in the pancreas that are damaged by an autoimmune response in type 1 diabetes. Islet transplantation is sometimes used to treat patients, but because the islets do not develop a robust blood supply when transplanted to accessible sites, such as the skin, they are instead infused into the liver, leading to difficulty in monitoring and loss of the islet graft.
“R-VECs’ capacity to vascularize human islets will lay the foundation to engineer long-lasting islets to potentially cure type I diabetes,” said co-author Dr. Joe Zhou, who was recruited as professor of regenerative medicine in medicine at WCM, and who collaborated with postdoctoral associate Dr. Ge Li on the islet studies. “Such vascularized islets would be more accessible, could have a better survival rate and might be superior to what we have now in clinical use. They could also offer new avenues to test drugs aimed at stopping the autoimmune response.”
“R-VECs also vascularize decellularized tissues, which serve as a native scaffold for organs. R-VECs engraft both in large and small vessels in decellularized scaffolds, increasing the survival of transplanted organs in mice,” said co-author Dr. Paolo de Coppi, National Institute of Health Research Professor, consultant paediatric surgeon at Great Ormond Street Hospital (GOSH) and head of Surgery, Stem Cells & Regenerative Medicine Section at the UCL Great Ormond Street Institute of Child Health in the UK. “This is an important advance since current engineered endothelia do not fully populate decellularized tissues, preventing long-term engraftment.”
R-VEC vessel networks are already being employed to study human diseases, such as uncovering how COVID-19 inflicts damage to organs. ”We are utilizing the R-VEC vascular network to investigate how the SARS-CoV-2 virus wreaks havoc on small blood vessels within organs, setting the stage to formulate new therapeutics” said Dr. Robert Schwartz, an assistant professor of medicine in the Division of Gastroenterology at WCM.
“The Organ-On-VascularNet system brings us one step closer to building large implantable organs with sufficient blood supply to perform daily functions. This technology also establishes a platform for pharmaceutical drug screening for malignant, metabolic and immune diseases and uncovering the pathways that determine organ-specific blood vessel diversity,” said Dr. Palikuqi.
“We see a new frontier in modern regenerative medicine as some of the major obstacles facing this field will now be challenged,” added Dr. Rafii, who is also the Arthur B. Belfer Professor in Genetic Medicine, chief of the Division of Regenerative Medicine and a member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine . “With this work, we have learned how to capitalize on endothelial cells’ reparative functions and can begin to tackle a variety of unmet medical needs.”
Dr. Shahin Rafii is the founder and a non-paid consultant to Angiocrine Bioscience. Dr. Robert Schwartz is a paid consultant for Miromatrix Medical Inc., a company focused on eliminating the organ transplant waiting list by bioengineering transplantable organs, including liver, kidney and heart.
news.weill.cornell.edu


