
Summary: A perspective in The American Journal of Clinical Nutrition challenges the “energy balance model,” which says weight gain occurs because individuals consume more energy than they expend. According to the authors, “conceptualizing obesity as a disorder of energy balance restates a principle of physics without considering the biological mechanisms underlying weight gain.” The authors argue for the “carbohydrateinsulin model,” which explains obesity as a metabolic disorder driven by what we eat, rather than how much.
- Public health messaging exhorting people to eat less and exercise more has failed to stem rising rates of obesity and obesity-related diseases.
- The energy balance model, which says weight gain is caused by consuming more energy than we expend, “restates a principle of physics without considering the biological mechanisms driving weight gain.”
- The carbohydrate-insulin model makes a bold claim: overeating doesn’t cause obesity; the process of getting fat causes overeating.
- The current obesity epidemic is due, in part, to hormonal responses to changes in food quality: in particular, high-glycemic load foods, which fundamentally change metabolism.
- Focusing on what we eat rather than how much we eat is a better strategy for weight management.
Statistics from the Centers for Disease Control and Prevention (CDC) show that obesity affects more than 40% of American adults, placing them at higher risk for heart disease, stroke, type 2 diabetes, and certain types of cancer. The USDA’s Dietary Guidelines for Americans 2020 – 2025 further tells us that losing weight “requires adults to reduce the number of calories they get from foods and beverages and increase the amount expended through physical activity.”
This approach to weight management is based on the century-old energy balance model which states that weight gain is caused by consuming more energy than we expend. In today’s world, surrounded by highly palatable, heavily marketed, cheap processed foods, it’s easy for people to eat more calories than they need, an imbalance that is further exacerbated by today’s sedentary lifestyles. By this thinking, overeating, coupled with insufficient physical activity, is driving the obesity epidemic. On the other hand, despite decades of public health messaging exhorting people to eat less and exercise more, rates of obesity and obesity-related diseases have steadily risen.
The authors of “The Carbohydrate-Insulin Model: A Physiological Perspective on the Obesity Pandemic,” a perspective published in The American Journal of Clinical Nutrition, point to fundamental flaws in the energy balance model, arguing that an alternate model, the carbohydrate-insulin model, better explains obesity and weight gain. Moreover, the carbohydrate-insulin model points the way to more effective, long-lasting weight management strategies.
According to lead author Dr. David Ludwig, Endocrinologist at Boston Children’s Hospital and Professor at Harvard Medical School, the energy balance model doesn’t help us understand the biological causes of weight gain: “During a growth spurt, for instance, adolescents may increase food intake by 1,000 calories a day. But does their overeating cause the growth spurt or does the growth spurt cause the adolescent to get hungry and overeat?”
In contrast to the energy balance model, the carbohydrate-insulin model makes a bold claim: overeating isn’t the main cause of obesity. Instead, the carbohydrate-insulin model lays much of the blame for the current obesity epidemic on modern dietary patterns characterized by excessive consumption of foods with a highglycemic load: in particular, processed, rapidly digestible carbohydrates. These foods cause hormonal responses that fundamentally change our metabolism, driving fat storage, weight gain, and obesity.
When we eat highly processed carbohydrates, the body increases insulin secretion and suppresses glucagon secretion. This, in turn, signals fat cells to store more calories, leaving fewer calories available to fuel muscles and other metabolically active tissues. The brain perceives that the body isn’t getting enough energy, which, in turn, leads to feelings of hunger. In addition, metabolism may slow down in the body’s attempt to conserve fuel. Thus, we tend to remain hungry, even as we continue to gain excess fat.
To understand the obesity epidemic, we need to consider not only how much we’re eating, but also how the foods we eat affect our hormones and metabolism. With its assertion that all calories are alike to the body, the energy balance model misses this critical piece of the puzzle.
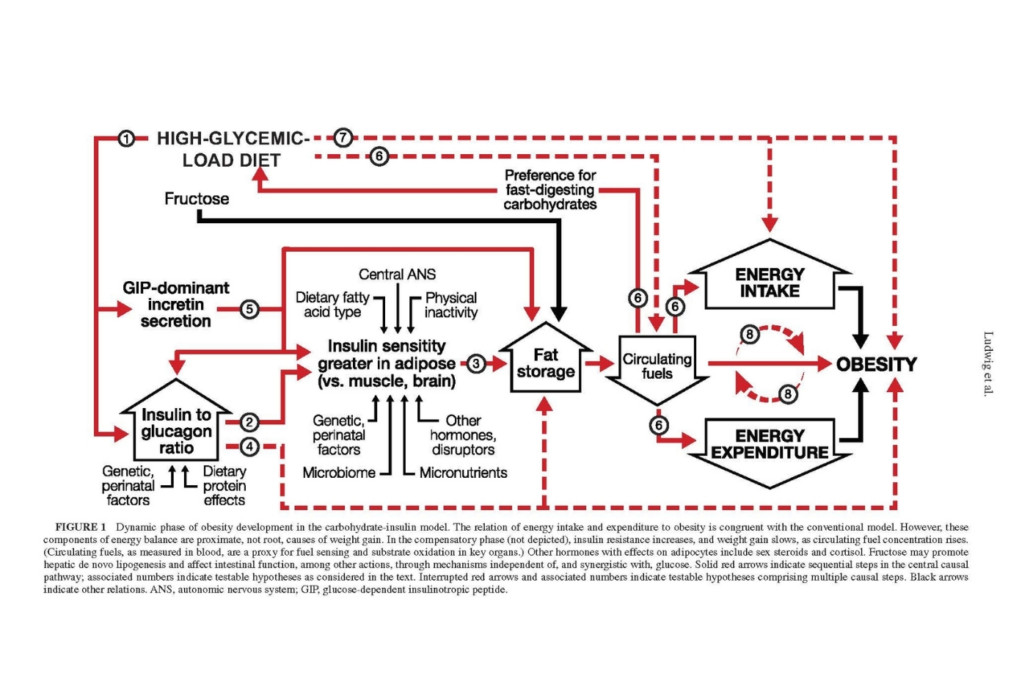
While the carbohydrate-insulin model is not new—its origins date to the early 1900s—The American Journal of Clinical Nutrition perspective is the most comprehensive formulation of this model to date, authored by a team of 17 internationally recognized scientists, clinical researchers, and public health experts. Collectively, they have summarized the growing body of evidence in support of the carbohydrate-insulin model. Moreover, the authors have identified a series of testable hypotheses that distinguish the two models to guide future research.
Adoption of the carbohydrate-insulin model over the energy-balance model has radical implications for weight management and obesity treatment. Rather than urge people to eat less, a strategy which usually doesn’t work in the long run, the carbohydrate-insulin model suggests another path that focuses more on what we eat. According to Dr. Ludwig, “reducing consumption of the rapidly digestible carbohydrates that flooded the food supply during the low-fat diet era lessens the underlying drive to store body fat. As a result, people may lose weight with less hunger and struggle.”
The authors acknowledge that further research is needed to conclusively test both models and, perhaps, to generate new models that better fit the evidence. Toward this end, they call for constructive discourse and “collaborations among scientists with diverse viewpoints to test predictions in rigorous and unbiased research.”


