 Tendons are bands of fibrous connective tissue that attach muscles to bones. They are soft tissues connected to stiff bones; this creates a complex interface with a very specific structure. Following injury, this structure is disrupted, and the connective tissue changes from a linear to a kinked formation. Excess scarring can also occur, changing the tendon’s mechanical properties and its ability to bear loads.
Tendons are bands of fibrous connective tissue that attach muscles to bones. They are soft tissues connected to stiff bones; this creates a complex interface with a very specific structure. Following injury, this structure is disrupted, and the connective tissue changes from a linear to a kinked formation. Excess scarring can also occur, changing the tendon’s mechanical properties and its ability to bear loads.
During the body’s natural healing processes, tendon and other cells are recruited to reconstruct the tendon’s original matrix of aligned connective tissue fibers. But this reconstruction can take weeks to months and the resultant tendon is often imperfect. This results in weakness, chronic pain and decreased quality of life.
Possible treatments for tendon injuries include tendon tissue grafts from patients or donors, but these pose risks such as infections, transplant rejection or necrosis. Synthetic transplants have been attempted, but mechanical, biocompatibility and biodegradation issues have hampered these efforts.
Another approach is to use mesenchymal stem cells (MSCs), specialized cells that play a pivotal role in tissue regeneration. At the wound site, they can differentiate into various cells types and produce signaling molecules which regulate immune response, cellular migration, and new blood vessel formation; this enables tissue regeneration.
However, treatment methods using systemic infusion, direct injection or genetic modification of MSCs present their own difficulties: infusion lacks targeting specificity to the injury site, direct injection requires prohibitively high cell numbers, and genetic modification is inefficient and produces cells that are difficult to isolate.
Yet another approach has been to construct biomaterial frameworks, or scaffolds, on which to introduce MSCs and growth factors in order to generate new tendon tissue. A collaborative team from the Terasaki Institute for Biomedical Innovation (TIBI) has utilized this approach to develop a method which has yielded significant improvements in MSC tendon regeneration.
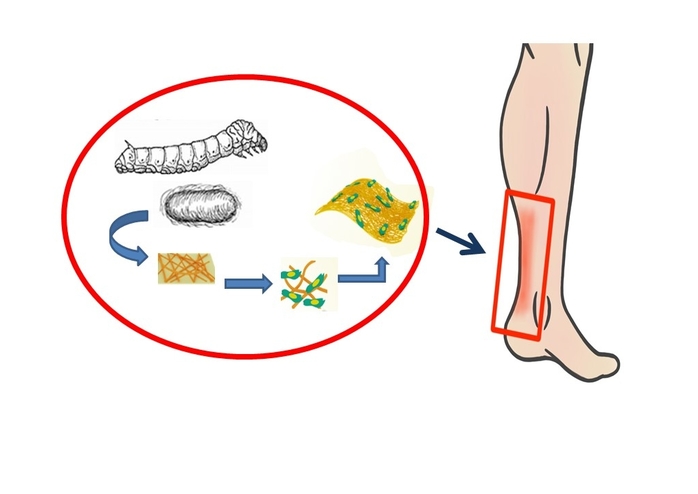
The team first turned to silk fibroin, a silk protein produced by the Bombyx mori silkworm. In addition to its use in beautiful silk fabrics, silk fibroin is used in optical and electrical devices, and in several biomedical applications, from suture materials to bioengineered ligaments, bone and even corneal tissue. Because of its superior strength, durability, biocompatibility and bio-degradative qualities, silk fibroin is ideal for use in scaffolds for tendons.
In order to improve the scaffold’s ability for tissue regeneration, the team next paired silk fibroin with GelMA, a gelatin-based, water-retaining gel, due to GelMA’s biocompatibility, controllable degradation, stiffness and ability to promote cell attachment and growth.
“The synergistic effects of GelMA’s capacity for supporting regenerative tissue formation and the structural advantages of silk fibroin make our composite material well suited for tendon repair,” said HanJun Kim, Ph.D., D.V.M, TIBI’s team leader on the project.
They prepared mixtures with varying ratios of silk fibroin and GelMA (SG) and fabricated them into thin nanofiber sheets. They then tested the sheets for fiber structure and stretchiness and chose an optimum formulation with the best mechanical properties. They also observed that the silk fibroin imparted an increased porosity to the material; this enhances tendon repair.
The optimized SG sheets were seeded with MSCs and subjected to various tests to measure MSC compatibility and differentiation, growth factor production, and genetic activity triggering matrix formation.
The MSCs on the SG sheets showed an increase in cell viability and proliferation over those on silk fibroin sheets without GelMA (SF). Genetic analysis showed that relevant gene activity in SG MSCs was significantly increased, in contrast to those on SF sheets, which was decreased.
Staining tests revealed that the MSCs on the SG sheets showed a more than 80% attachment rate and had an elongated shape characteristic of cells attached to a surface, as opposed to a 60% attachment rate, with spherically-shaped cells observed on SF and GelMA only surfaces.
Further tests on a growth factor secreted by MSCs seeded onto nanofiber sheets showed that the growth factors produced by the MSCs on the SG sheets were best able to repair injured tendon tissue cultivated in a culture dish.
Experiments were also conducted on live rats with injured Achilles tendons. MSC-seeded nanofiber sheets were implanted onto the injury site and the SG sheets promoted the most accelerated healing, with reduced injury sites and the formation of well-aligned, densely packed tendon fibers and remodeled muscle components.


