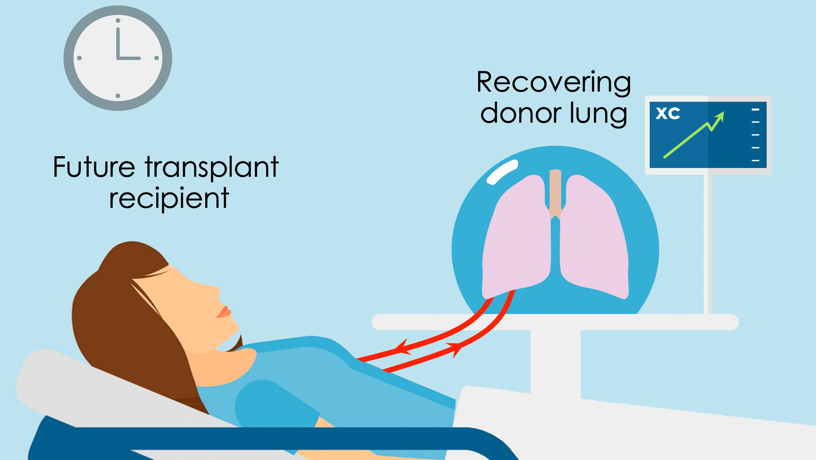
Illustration of envisioned application of cross-circulation to expand the number of organs that can be used for life-saving transplantations.
Respiratory disease is the third leading cause of death worldwide, and lung transplantation is still the only cure for patients with end-stage lung disease. Despite advances in the field, lung transplantation remains limited by the low availability of healthy donor organs, and most donor lungs cannot be used due to severe but potentially reversible injuries. Currently, a method known as ex vivo lung perfusion (EVLP) is used to provide lung support outside the body and recover marginal quality donor lungs before transplantation. However, EVLP provides only a limited duration of six to eight hours of support—a time that is too short to recover the majority of severely damaged donor lungs.
A multidisciplinary team from Columbia Engineering and Vanderbilt University has now demonstrated that severely injured donor lungs that have been declined for transplant can be recovered outside the body by a system that uses cross-circulation of whole blood between the donor lung and an animal host. For the first time, a severely injured human lung that failed to recover using the standard clinical EVLP was successfully recovered during 24 hours on the team’s cross-circulation platform. The study is published today in Nature Medicine.
The investigators, led by Gordana Vunjak-Novakovic, University Professor and The Mikati Foundation Professor of Biomedical Engineering and Medical Sciences at Columbia Engineering, and Matthew Bacchetta, Surgical Director of the Vanderbilt Lung Institute, attributed the accomplishment of their major milestone to the physiologic milieu and systemic regulation that their unique platform provides to explanted human lungs.
“It is the provision of intrinsic biological repair mechanisms over long-enough periods of time that enabled us to recover severely damaged lungs that cannot otherwise be saved,” said the study’s lead authors, Ahmed Hozain (surgical research fellow at Columbia Engineering) and John O’Neill (adjunct associate research scientist at Columbia Engineering).
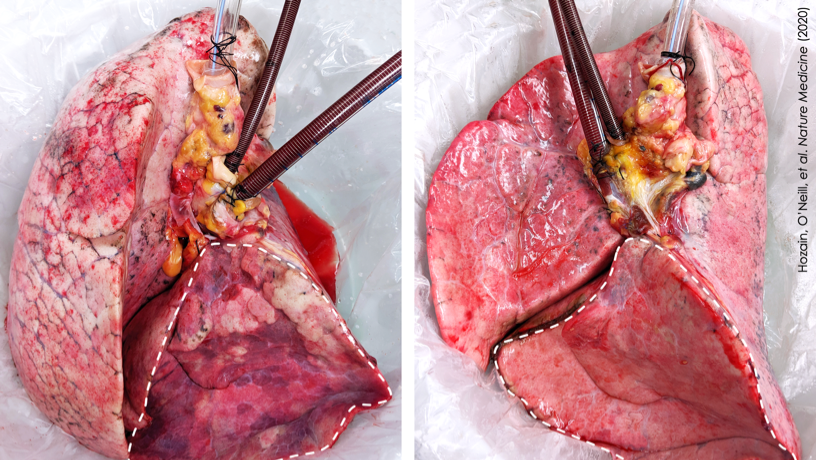
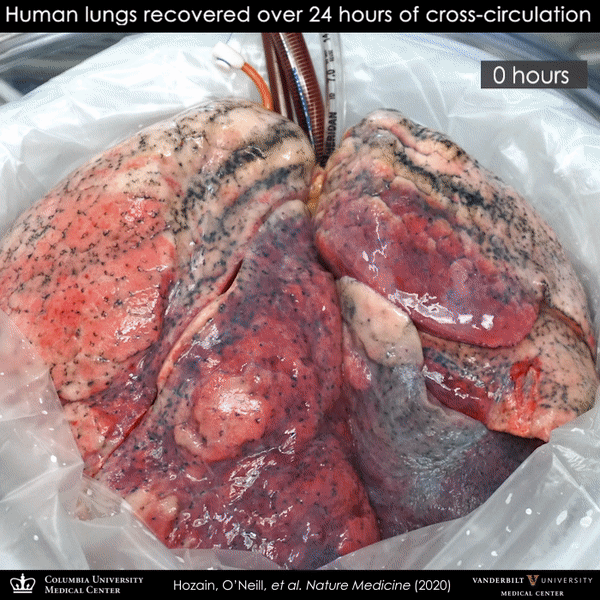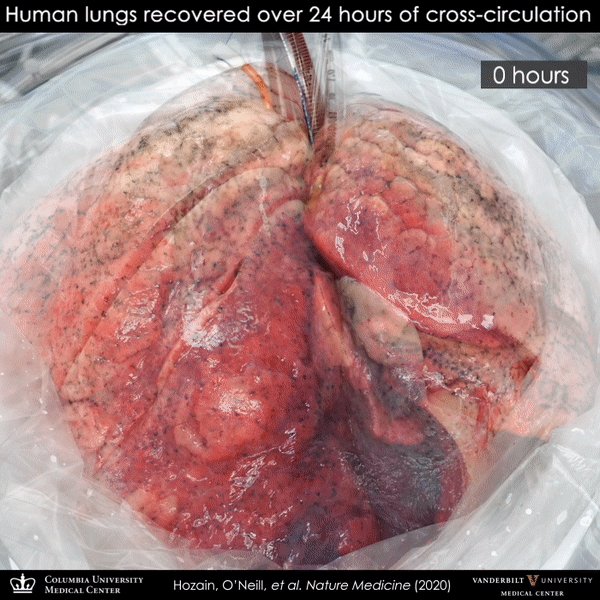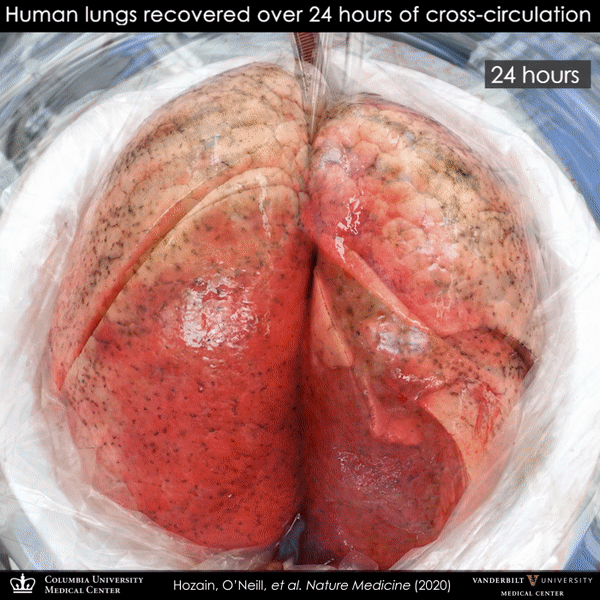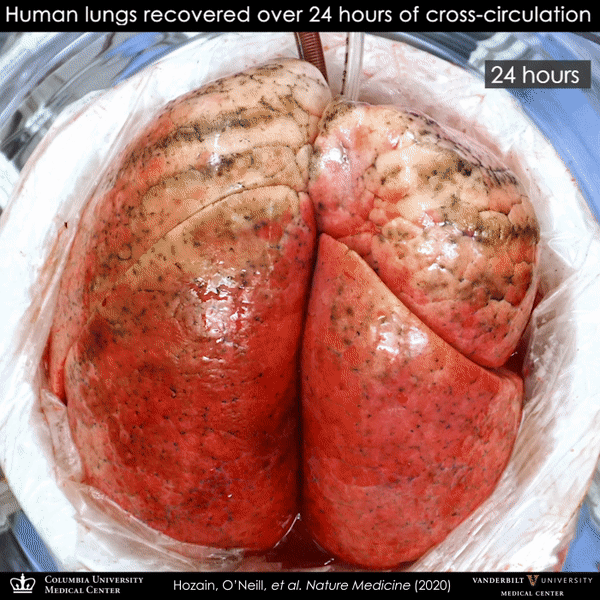
Human lung that failed on EVLP (left) and then recovered on cross-circulation (right).
Over the past eight years, the researchers have been developing their radically new method to provide more lungs for patients in dire need of organ transplantation. In 2017, they demonstrated the feasibility of cross-circulation support of whole lungs outside the body. In 2019, they demonstrated the efficacy of cross-circulation by regenerating severely damaged swine lungs, and in 2020, they successfully extended the duration of cross-circulation support to an unprecedented four days.
Now, in this new paper, the team shows that explanted human lungs, already declined for transplantation, can be recovered on their cross-circulation platform, which successfully maintained lung integrity and resulted in functional lung recovery. Throughout the 24 hours of cross-circulation, the team saw substantial improvements of cell viability, tissue quality, inflammatory responses and—most importantly—respiratory function.
“We were able to recover a donor lung that failed to recover on the clinical ex vivo lung perfusion system, which is the current standard of care. This was the most rigorous validation of our cross-circulation platform to date, showing great promise for its clinical utility,” Vunjak-Novakovic said.
This particular donor lung demonstrated persistent swelling and fluid buildup that could not be resolved, and it was declined for transplantation by multiple transplant centers and eventually offered for research. By the time the team received this lung, it had experienced two periods of cold ischemia that totaled 22.5 hours, plus five hours of clinical EVLP treatment. Remarkably, after 24 hours on cross-circulation, the lung showed functional recovery.
Video showing the sequence of lung recovery on cross-circulation system.
Vunjak-Novakovic noted that the size and profile of their multi-institutional research team—25 investigators with expertise in bioengineering, surgery, immunology, stem cells, and various clinical disciplines—reflects the complexity of this translational project.
Zachary Kon, Director of Lung Transplantation Program, NYU Langone Health, who was not involved in the study, commented: “As a lung transplant surgeon, I have seen many patients not receive lung transplants they desperately needed. I find this work intriguing and hope this technology will make more donor lungs available."
The investigators emphasize that more work needs to be done before cross-circulation can become a clinical reality. For clinical application of the cross-circulation platform, they envision two clinical scenarios for application of the cross-circulation platform, which they are planning to pursue. One approach is to directly translate the method demonstrated in this new study, with the human donor lung recovered by “xenogeneic” cross-circulation with a medical-grade, pathogen-free animal host. To this end, the safety, feasibility, risk profiles, and outcomes of xenogeneic cross-circulation will need to be evaluated in large numbers of lungs.
Another approach is that critically ill patients already awaiting transplantation on artificial lung support could serve as the cross-circulation host to recover an injured donor lung, which they would receive for transplant as soon as the organ recovers. As described in the paper, the xenogeneic cross-circulation platform may also serve as a research tool to investigate organ regeneration, transplant immunology, and the development of novel therapeutics.
Looking ahead, the researchers hope to extend the benefits of their cross-circulation platform to the recovery of other human organs, including livers, hearts, kidneys, and limbs.
BY HOLLY EVARTS


