The study, published Feb. 16 in Cell, reveals a novel set of molecular signals connecting fungi in the gut to their host’s cells throughout the body, including immune cells and neurons.
“We have made a direct link between a major immune pathway induced by fungi in the lining of the intestine and signals in the nervous system that impact animal behavior,” said senior author Dr. Iliyan Iliev, associate professor of immunology in medicine in the Division of Gastroenterology and Hepatology and a member of the Jill Roberts Institute for Research in Inflammatory Bowel Disease at Weill Cornell Medicine.
Drs. Dilek Colak, Melanie Johncilla and Megan Allen from Weill Cornell Medicine and Dr. Rhonda K. Yantiss from Weill Cornell Medicine and NewYork-Presbyterian also contributed to this study.
The lining of the intestine must balance conflicting needs, absorbing water and nutrients from food while acting as a barrier to prevent the vast population of microbes in the gut from invading the bloodstream.
Examining this system in a mouse model, the scientists mapped the locations of different fungi within the intestine and found that a unique consortium of fungi tends to accumulate at specific sites near the gut epithelium, or lining, suggesting that these species have colonized the gut and interact closely with the nearby epithelial cells.
Mice carrying some of these fungi enjoyed better protection against events that can disrupt the intestinal barrier, such as intestinal injury and bacterial infection. “There was fortification of those barrier functions when we added that specific fungal community to mice,” Dr. Iliev said.
Improving intestinal barrier integrity wasn’t the only effect of the fungi. In separate experiments, the team found that mice carrying the fungal community in their gut displayed more social behavior than animals without these fungi.
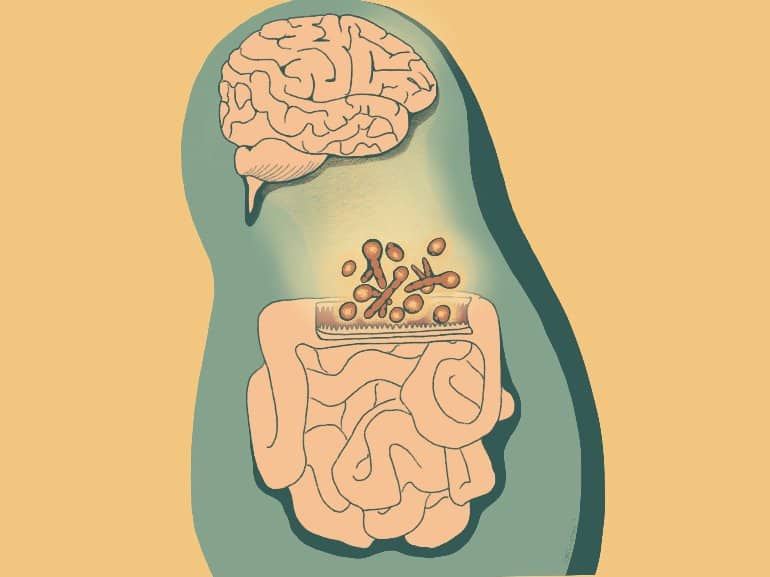
“There is this harmony—a kind of communication between or across different types of organisms,” Dr. Iliev said.
Now the investigators hope to explore that communication network further. “We are trying to go deeper into the mechanisms of this interaction, looking at the signals that are involved at the neuronal level in different brain regions,” said lead author Dr. Irina Leonardi, an instructor of immunology in medicine in Dr. Iliev’s laboratory at the Jill Roberts Institute for Research in IBD.
One tantalizing possibility is that different microbial communities in the gut could stimulate different regions of the brain and the immune system, exerting distinct effects on their hosts’ biology.
“This opens a whole new area to explore,” Dr. Iliev said.


