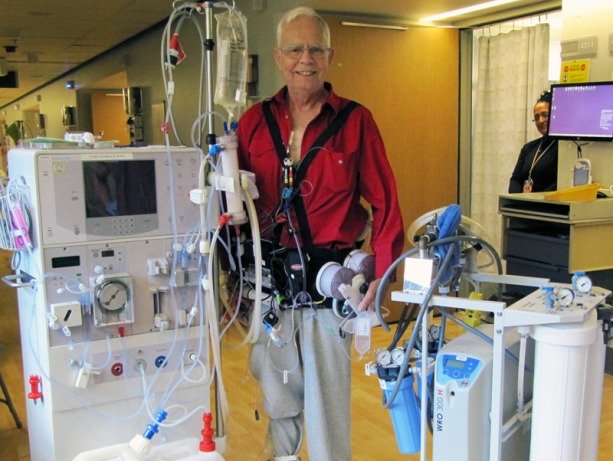
The results of an exploratory clinical trial indicate that a wearable artifical kidney could be developed as a viable, new dialysis technology. Some redesigns would be required to overcome
The findings are reported June 2 in JCI Insight, a
The technology may become an alternative to conventional hemodialysis for people with
The Food and Drug
A collaboration with the
Those leading the
The trial was conducted to determine the safety and efficacy of the device — its ability to take over some functions of failed kidneys. The researchers also wanted to ask the participants about their impressions of the experimental treatment, and to compare those with standard dialysis treatment.
In the patients studied, the device was shown to effectively clear the blood of waste products, like urea, creatinine and phosphorus while removing excess water and salt. These are normally filtered out and eliminated by working kidneys.
The usual diet for patients on standard dialysis is highly limited. In contrast, on the Wearable Artificial Kidney, the patients’ blood electrolytes — like sodium and potassium — and their blood fluid volume remained balanced during the test, even without any diet restrictions. Regulating the volume and composition of body fluids is another job of normal kidneys.
During the trial, the participants tolerated the treatment well and did not have any serious, adverse effects. The circulatory system, which keeps blood moving throughout the body, stayed stable in all the patients.
However, the trial was stopped after the seventh patient because of technical problems with the device. These included the excessive formation of carbon dioxide gas bubbles in the dialysis solution, and intermittent variations in solution and blood flow.
These technical complications, according to the researchers report, will need to be addressed through device redesign and refinement to enhance safety and reliability prior to any further,
The patients participating in the study reported greater satisfaction during their treatment with the Wearable Artificial Kidney when compared to their ratings of care during conventional dialysis center treatment. Previous studies have shown that patients and their families highly value treatments that can be given at home and that increase independence and the freedom to travel.
«I was amazed at how well it worked for me. It was heavy and cumbersome, but I’d be wearing it today if I could. It just gives you so much more freedom," said one of the patients in the trial, Chuck Lee, in describing his experience in a Nov. 7, 2015, news article, «Wearable Artificial Kidney earns
The researchers and inventors noted that redesign of the WAK components will be directed at the ease of use and reliability of operation of the device, because the ultimate goal is for treatment to be administered independently at home by the patient or their caregivers.
The patient trial of the device was supported by an unrestricted gift from the
Source: http://hsnewsbeat.uw.edu/story/patient-trial-confirms-wearable-artificial-kidney-proof-concept


