 Their secret? Starting from scratch — with computational techniques that let them explore more than four trillion different chemical interactions.
Their secret? Starting from scratch — with computational techniques that let them explore more than four trillion different chemical interactions.
In a new study — published online Aug. 17, 2016, in Nature — the researchers used the newly deciphered atomic structure of the brain’s «morphine receptor» to
More work is needed to establish that the newly formulated compound is truly
Deaths from opioid drug overdoses have been on the rise in the US for decades. According to the Centers for Disease Control and Prevention, 28,000 Americans died of narcotic overdoses in 2014, four times more than in 1999, with more than half of these deaths involving prescription drugs. The epidemic has gotten the attention of national leaders: in February, 2016, President Obama proposed $1.1 billion in new funding for opioid addiction treatment, and in July Congress passed the Comprehensive Addiction and Recovery Act, a bill intended to curb opioid abuse and improve treatment.
But as damaging as opioids can be, modern medicine depends on these drugs as our most powerful weapon against pain.
«Morphine transformed medicine," said Brian Shoichet, PhD, a professor of pharmaceutical chemistry in UCSF’s School of Pharmacy and
Virtual Experiments Lead to Novel Opioid Chemistry
Much of drug discovery, Shoichet says, begins by taking a successful drug like morphine and tweaking its structure to try to get rid of side effects while maintaining its primary function. The new study took a different, much more radical approach: «We didn’t want to just optimize chemistry that already existed," Shoichet said. «We wanted to get new chemistry that would confer completely new biology.»
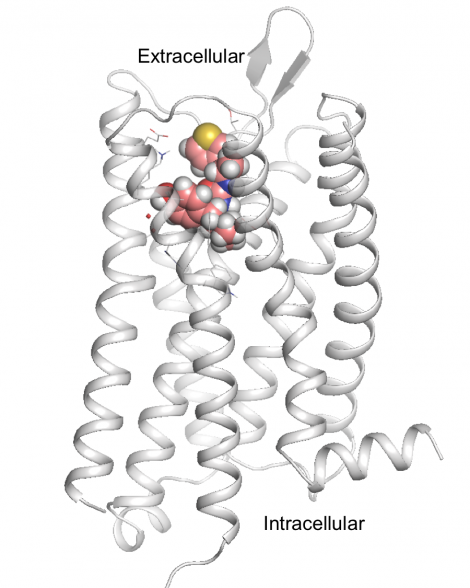 Key to the new paper was knowing the atomic structure of the
Key to the new paper was knowing the atomic structure of the
«With traditional forms of drug discovery, you’re locked into a little chemical box," Shoichet said. «But when you start with the structure of the receptor you want to target, you can throw all those constraints away. You’re empowered to imagine all sorts of things that you couldn’t even think about before.»
With this structural information in hand, Shoichet’s team turned to a computational approach called molecular docking, which was pioneered in the 1980s at UCSF’s School of Pharmacy by Shoichet’s mentor, emeritus professor Tack Kuntz, PhD. In a
This led to a
Only then did the team actually test these candidate drugs in the real world.
‘Unprecedented, Weird and Cool’ New Biology
In further pharmacological tests conducted in the Roth lab, PZM21 exhibited the «new biology» the researchers had been looking for: efficiently blocking pain without producing the constipation and breathing suppression typical of traditional opioids. In addition, PZM21 appeared to dull pain by affecting opioid circuits in the brain only, with little effect the on opioid receptors in the spinal cord that mediate pain reflexes. No other opioid has such a specific effect, Shoichet said, calling it «unprecedented, weird and cool.»
Additional behavioral tests in mice suggested the drug may also lack the addictive qualities of existing opioids. Specifically, the drug didn’t produce the hyperactivity other opioids trigger in mice by activating the brain’s dopamine systems — which are also involved in addiction. Perhaps more tellingly, mice did not spend more time in test chambers where they had previously received doses of PZM21 — a test called «conditioned place preference» that is considered a correlate of human
 «We haven’t shown this is truly
«We haven’t shown this is truly
The study is a successful example of the
«This promising drug candidate was identified through an intensively
«If you took away any one of these collaborators it simply wouldn’t have worked," Shoichet added. «Without Kobilka’s structure, our computation, Roth’s pharmacology, and Gmeiner’s ability to put an atom in exactly the place you want it, this never would have been possible.»
Lead authors on the new paper were Aashish Manglik, MD, PhD, of Stanford University School of Medicine; Henry Lin, PhD, of the UCSF School of Pharmacy; and Dipendra K. Aryal, PhD, of the UNC School of Medicine. Lin is now principal scientist at The Janssen Pharmaceutical Companies, a division of Johnson & Johnson. Manglik, Lin, Gmeiner, Kobilka, Roth, Shoichet, and
The research was supported by the US National Institutes of Health grants GM106990 (B.K.K., B.K.S. and P.G.), DA036246 (B.K.K.), GM59957 (B.K.S.), and the National Institutes of Mental Health Psychoactive Drug Screening Program (B.L.R.) and DA017204 (B.L.R., D.A.), DA035764 (B.L.R.) and the Michael Hooker Distinguished Professorship (B.L.R.) and the German Research Foundation Grants Gm 13/10 and GRK 1910 (P.G). H.L. received a
UCSF is a leading university dedicated to promoting health worldwide through advanced biomedical research,
Source: http://www.ucsf.edu/news/2016/08/403836/researchers-develop-safer-opioid-painkiller-scratch


