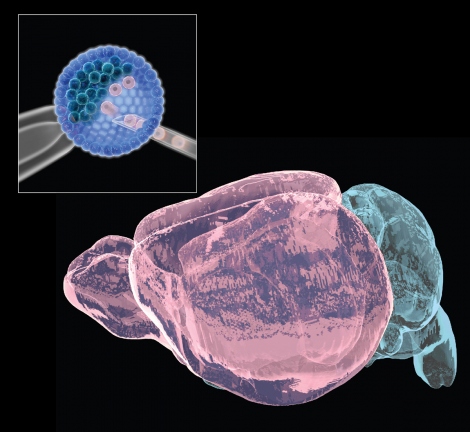
The authors aim to use this technique to learn more about how brain cancers form, about how genetic alterations in different parts of the brain impact diseases such as autism or schizophrenia. They developed a new and improved mouse “chimera” by clearing out a space in the developing brains of early-stage mouse embryos, enabling cells derived from other mice to grew there instead.
To scientists who study how mammals develop through embryogenesis and beyond, a chimera is a genetically retooled mouse combining cells from different mice. Chimeras are used to sort out key questions about how organs emerge and function over time and how genes behave differently in different parts of an animal.
In the brain, for instance, researchers might want to ask whether a specific gene variant that has been linked to schizophrenia plays different roles in “executive” regions of the brain’s prefrontal cortex versus deeper, more primal brain regions. However, with conventional methods many complex steps, including mouse breeding, are required to get answers about how specific genes and the proteins act in different parts of the brain.
The researchers, led by Bjoern Schwer, MD, PhD, assistant professor of neurological surgery at UCSF, and Fred Alt, PhD, who directs the Program in Cellular and Molecular Medicine at Boston Children’s, developed a new mouse strain in which cells that form key brain structure were programmed to poison themselves with a bacterial toxin during the earliest stages of brain development. The death of these cells left space for injected embryonic stem cells from another mouse to regrow the missing brain structures, resulting in a mouse in which different parts of the brain can have totally distinct genetics.
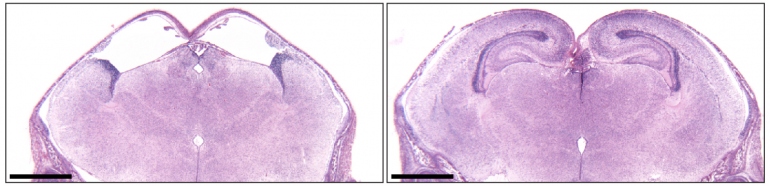
Schwer and Alt, who are co-senior authors of an October 10, 2018 Nature paper published detailing the new technique, believe their new technique will speed new discoveries to help understand human brain maladies.
“Mice with embryonic-stem-cell-derived brain regions are indistinguishable from normal mice in memory and learning tasks,” said Schwer, who is a member of the UCSF Weill Institute for Neurosciences and the Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research at UCSF. “Now we can use embryonic stem cells to reliably generate key structures of the brain, arguably the most complex organ in vertebrates.”
With the technique, called neural blastocyst complementation, researchers can routinely make one or more genetic alterations in embryonic stem cells and directly observe their effects in the brain, according to Schwer.
Alt, who is a Howard Hughes Medical Institute (HMMI) investigator, invented blastocyst complementation earlier to study the development of T and B lymphocytes in the immune system, but until now nobody had made it work to generate brain structures.
“We think of this strategy as a completely new platform for neurobiologists to study every aspect of the brain,” Alt said, “from basic knowledge of which genes control brain development to potentially finding new gene therapies for brain cancers and psychiatric disorders.”
https://www.ucsf.edu/news/2018/10/411981/building-patchwork-brain-study-neurological-disease


