The nanosystem — «diagnostic nanoprobe» — consists of brightly fluorescent semiconductor particle of several nanometres’ size and special molecules («single-domain antibodies») connected with its surface, capable of detecting cancer cells of a certain kind and connecting with them, making them «visible». The nanosystem uses only antibodies from organisms of lamas, camels and sharks, because compared to antibodies from other animals’ organisms they have a simpler structure and a smaller size.
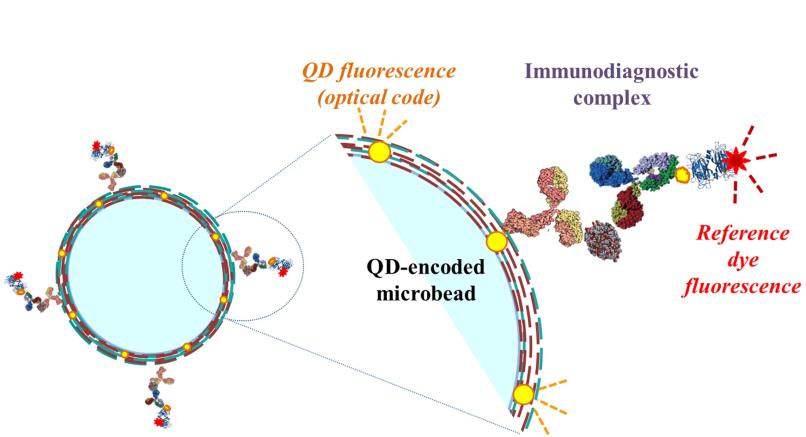
Korean researchers have synthesized semiconductor nanoparticles, able to shine in the infra-red spectral band. Russian scientists managed to connect single-domain antibodies with the nanoparticle surface. As the result, the highest efficiency of cancer cells’ detection has been reached in animal experiments.

«The main advantage of the new nanoprobe is its small size — the diameter of created diagnostic labels is 13 times less than that of existing analogues — and high stability (the nanoprobe doesn’t decompose at high temperatures) along with the specificity of the detection of cancer cells’ presence», said the leading scientist of MEPhI interdepartmental Laboratory of Nano-Bioengineering Igor Nabiev.
He added that «the fluorescence of the nanoparticles in the infra-red band allows use the transparent region of biological tissues, which, together with nanoprobe’s high light intensity allows see it even in the deep depth of penetration, detecting cancer cells practically in every part of the human organism.»
According to the scientist’s words, the new diagnostic system uses molecules detecting cells of breast and prostate cancer. However, the usage of the similar technology with the application of other detecting molecules will allow diagnose other oncological, infectious, phlogistic and immune diseases. Apart from diagnostics the probes can be applied for the D2D of medicines.
The work on the diagnostic nanoprobe as a part of the joint Russian-Korean project is conducted with the support of the government of the RF. Scientists are currently at the final stage of the technology development, the applications for 2 patents have been made. The incorporation of the new diagnostic system into medical practice is expected to be made in 2–3 years after the end of the project and the completion of pre-clinical and clinical trials.
Source:
https://mephi.ru/eng/content/news/1812/116710/




