“PAD is very common in diabetic patients, but it is difficult to diagnose because patients experience symptoms when the disease is already at an advanced state,” said study leader Wawrzyniec Lawrence Dobrucki, a professor of bioengineering and of medicine and head of the Experimental Molecular Imaging Laboratory at the Beckman Institute for Advanced Science and Technology. “When the PAD is left untreated, it often leads to foot ulcerations and limb amputations – thousands every year in the United States. It’s a serious, costly and debilitating disease.”
In PAD, arteries in the limbs become narrowed, causing pain and limiting mobility due to lack of blood and oxygen. Treatment options for PAD are few. Some medications aim to increase perfusion in the limbs but are not very effective, Dobrucki said. Doctors recommend exercise, but it is painful and difficult for PAD patients, and other cardiovascular complications may make physical activity impractical. Many patients require surgery to place vascular stents or an arterial bypass.
Dobrucki’s group partnered with Marni Boppart, a professor of kinesiology and community health, to study the effects of stem cells injected at the site where the artery narrows. Boppart is an expert in mesenchymal stem cells, which play a role in injury healing and tissue regeneration in muscle and other tissues. Other research has focused on various individual growth factors to promote blood vessel formation – a process called angiogenesis – but none have worked well.
“Angiogenesis is a sophisticated process involving many growth factors and interactions between key proteins,” Dobrucki said. “That’s why we used a more nature-inspired therapy to promote angiogenesis in PAD-affected limbs and used the stem cells. They know what to release, how much and when to stop, for example. They also respond to and modify the tissue microenvironment to optimize their therapeutic effect.”
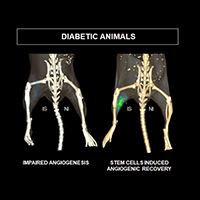
Positron emission tomography imaging monitors blood vessel formation in mice. Diabetic mice injected with mesenchymal stem cells showed angiogenic processes resembling that of nondiabetic animals.
Image courtesy of W. Lawrence Dobrucki
The researchers surgically narrowed the femoral artery in one leg of diabetic mice. They injected MSCs, taken from the muscles of young mice, into the legs of the experimental mice, while the control group got a saline injection. Then they used noninvasive imaging techniques, developed in Dobrucki’s lab, to monitor blood flow and blood vessel formation in the mice, comparing both the experimental and control groups and the affected and nonaffected legs in each mouse.
“We clearly demonstrated the capacity for MSCs to increase angiogenesis, peripheral perfusion and muscle function,” Boppart said. “We saw that MSCs promoted muscle healing by creating new vessels in the tissue that compensated for restricted blood flow. MSC transplantation provides the opportunity to maximize vessel growth in PAD to maintain or rejuvenate skeletal muscle.”
The researchers also performed an analysis of gene expression within the tissue and found that, in mice given the stem cell injections, the gene expression in the leg with PAD was close to that of the unaffected leg. They also found that, compared with the mice that did not get the stem cells, there were genes activated to combat some of the diabetic complications – for example, genes associated with inflammation were repressed.
“Our results suggest that stem cell treatment could be used for those patients at severe stages of PAD who cannot exercise,” Dobrucki said. “Stem cell treatment could help bring them to the level where they can start exercising, or it could save an extremity before it needs amputation.”
Next, the researchers are hoping to identify subpopulations of MSCs that demonstrate the most potential to treat PAD, as well as optimizing the conditions to isolate these rare cells from human fat tissue and skeletal muscle. They are also studying how long the MSCs stay active after injection, and what kind of immune response they may trigger.
“We also are attempting to develop an acellular approach to rejuvenation of muscle repair and growth,” Boppart said. “We hope to identify the optimal composition of factors that the cells release, as well as the medium for release. Treatment with the right combination of factors, rather than the cells themselves, provides the opportunity to circumvent the potential for rejection by the immune system.”
The National Institutes of Health, the AHA Scientist Development Grant and the Arnold Beckman Foundation supported this work.
https://news.illinois.edu/blog/view/6367/575544


