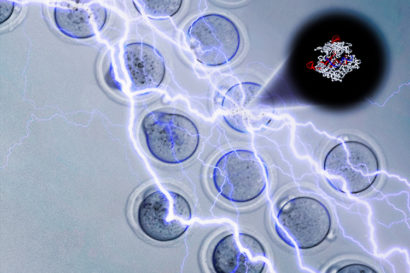
Electroporation jolts embryos with electricity to open holes that allow the CRISPR-Cas9 molecule (upper right) to enter and edit the DNA. Andrew Modzelewski, Lin He image.
To do that, they need to knock out or modify specific genes in lab animals — in particular, mice — and see what goes awry. Today’s gold standard for creating these «knockouts» or «knockins» is to edit the genes inside mouse embryonic stem cells, use these cells to create mosaic mice, and then crossbreed the mice to get a pure genetic strain. Because of
The new method, called
«The key fundamental insights about the biological significance of a gene usually come from in vivo
The new method, described in a paper in press at the Journal of Biological Chemistry, gets around a
The UC Berkeley researchers found that a simple lab technique called electroporation works much better, allowing them to insert

Using a micropipette, researchers suction up embryos in preparation for inserting CRISPR-Cas9 by electroporation. Lin He image.
«In the not too distant past, it would cost at least $25,000 and take at least 6 months to make a knockout mouse," said Russell Vance, a UC Berkeley professor of molecular and cell biology and director of the Cancer Research Laboratory, where the transgenic mouse work was performed. «With CRISPR, and improvements such as
The UC Berkeley group is now working with several transgenic mouse facilities in hopes that they will adopt and improve this electroporation technique, which she suspects will also simplify the creation of other transgenic mammals.
Knockouts require IVF team
Creating transgenic mice requires a skilled in vitro fertilization team. Technicians use hormone injections to prepare the females for mating, after which they harvest the fertilized eggs and, before the eggs start to divide, inject them one at a time using a fine needle. Currently, technicians inject two RNA molecules — messenger RNA (mRNA), which codes for the Cas9 protein, and guide RNA, which provides the address for
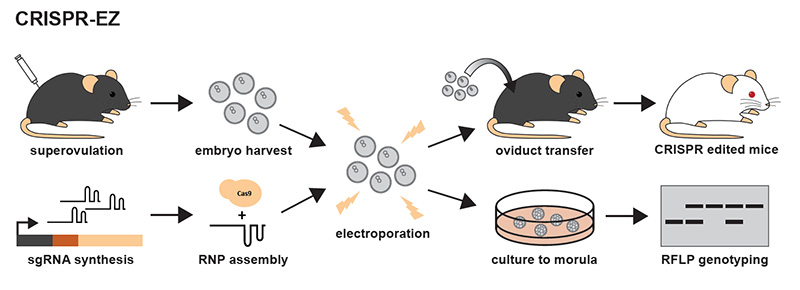
Creating transgenic mice involves harvesting fertilized eggs and electroporating them to insert the CRISPR-Cas9 molecule, then implanting the edited embryos in another mouse. The targeted gene is linked with a gene that produces white fur, making it easy to see which mice have been successfully edited.
The engineered embryos are implanted into a falsely pregnant mouse, where they gestate for about 20 days before birth. Given the inevitable embryo deaths during injection and the failure of the embryos to implant or go to term, the
«The actual percentage of live births from injected embryos is around 10 to 15 percent for most transgenic facilities, which is a problem with the procedure," she said. «Sometimes people collect more than 100 embryos just to generate one or two mice with desirable gene editing.»
Electroporation appears to do less damage to the embryos than microinjection: between 30 and 50 percent of the embryos resulted in live births.
Apparently, too, inserting
For a more complex procedure — modifying a short DNA sequence within a gene — the method was successful 42 percent of the time in a pilot experiment.
«With such a high success rate, you can use this to test your guide RNAs very quickly," she said. «If your
The high embryo viability and very high
Accidental discovery
In her lab, He studies small bits of RNA called microRNA, which modify how DNA is transcribed and thus control important processes inside a cell. Some types of cancer have been linked to problems with miRNA.
Using a micropipette, researchers suction up embryos in preparation for inserting CRISPR-Cas9 by electroporation. Lin He image.
«It has worked like magic ever since," He said. «You would never think that this would work, because Cas9 is a gigantic molecule. I was surprised that such a huge protein could be electroporated efficiently.»
While most universities have transgenic labs where microinjections are performed, electroporation simplifies the procedure enough that individual labs might eventually do it themselves, she predicted.
Graduate student Sean Chen and postdoctoral fellow Andrew Modzelewski spearheaded this study, Other
This work was supported by the National Cancer Institute (R01 CA139067) and National Institute of General Medical Science (RO1 1R01GM114414) of the National Institutes of Health.
Source: http://news.berkeley.edu/2016/05/27/faster-more-efficient-crispr-editing-in-mice/


