 This new compound can find and help remove mutant mitochondria DNA from cells. (Ⓒ Mindy Takamiya/Kyoto University iCeMS)
This new compound can find and help remove mutant mitochondria DNA from cells. (Ⓒ Mindy Takamiya/Kyoto University iCeMS)Mitochondria perform many cell functions, including energy production, biomolecule synthesis and calcium homeostasis. Mitochondria have their own DNA, different from the DNA found inside the cell’s nucleus. Mutations in this mitochondrial DNA can lead to diseases, causing blindness, muscle weakness and even death.
In some mitochondrial diseases, mutated DNA and normal DNA co-exist. “This state is called heteroplasmy,” explains Kyoto University’s Takuya Hidaka, the first author of the study. “Mitochondrial function can be maintained by normal mitochondrial DNA when the heteroplasmy level is low. But it is impaired when mutated DNA exceeds a critical threshold. To cure mitochondrial diseases, we need to be able to remove mutant mitochondrial DNA from cells.”
Current approaches for such mitochondrial diseases are problematic, explains iCeMS bioengineer Ganesh Namasivayam Pandian, who led the study. Some involve injecting genetic material into cells, which could lead to unwanted alterations. In others, antioxidant drugs are administered to reduce the impacts of the mutant DNA, without addressing the core mutation.
Pandian worked with iCeMS chemical biologist Hiroshi Sugiyama, Takuya Hidaka and colleagues to develop a chemical-based approach that overcomes these issues.
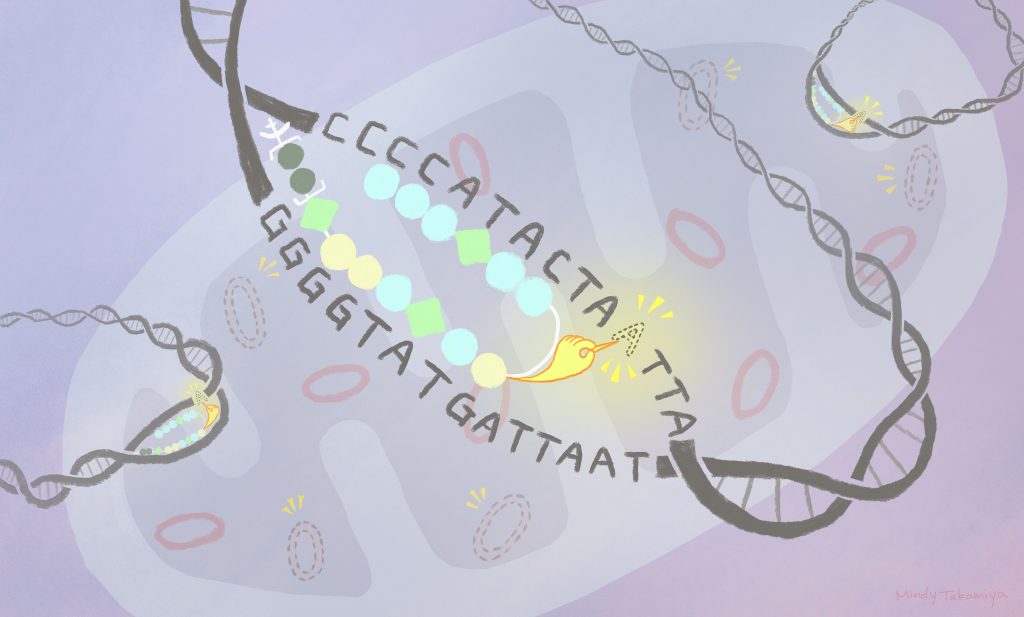
They designed a compound made of a mitochondria-penetrating peptide (MPP) bound to a polymer, called a pyrrole-imidazole polyamide (PIP), which can be modified to target a specific DNA sequence. These were then attached to an existing anti-cancer drug, called chlorambucil.
The MPP takes the compound into the mitochondria, where the PIP binds to its target DNA sequence. This allows the chlorambucil to damage the targeted DNA for elimination. Analyses showed the technique reduced the amount of mutant mitochondrial DNA in lab cultures of human cells. Further research using disease models is needed to determine the approach’s effectiveness inside a living organism.
By adapting the chemical make-up of the PIP, the therapeutic compounds can be programmed to attach to different DNA sequences, and so target a range of mitochondrial genetic diseases.
“Our proof-of-concept study can be extended to mitochondrial mutations that cause diseases like Leber’s hereditary optic neuropathy, an inherited form of vision loss that currently has no proven treatment,” says Pandian.
icems.kyoto-u.ac.jp


