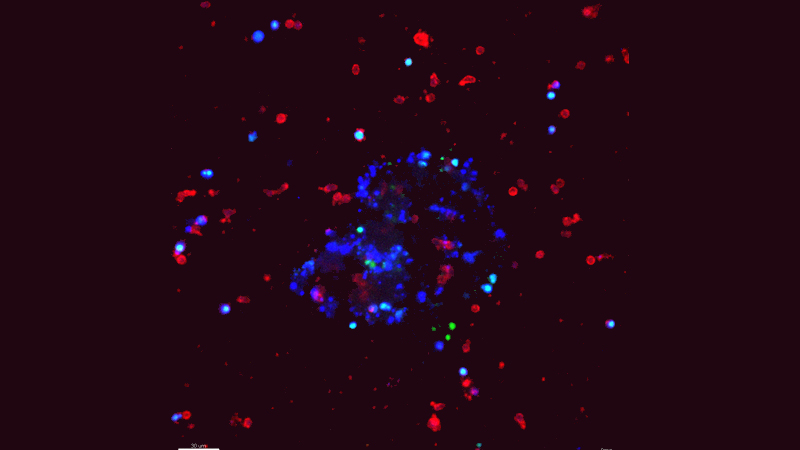
A blue tumor organoid surrounded by red NK cells Credit: Isaac Chan, M.D.
They propose that therapies targeting this process could be developed to halt or prevent metastasis and reduce breast cancer deaths.
Natural killer (NK) cells, a type of immune cell, are known to limit metastasis by inducing the death of cancer cells. But metastases still form in patients, so there must be ways for cancer cells to escape. Using a novel cell culture method developed by lead author Isaac Chan, M.D., Ph.D., a medical oncology fellow at Johns Hopkins working in the the laboratory of Andrew Ewald, Ph.D., the researchers studied the interactions between NK cells and invasive breast cancer cells in the laboratory in real time. They discovered that metastatic breast cancer cells can reprogram NK cells so that they stop killing cancer cells and, instead, assist in metastasis.
This work, published July 9 in the Journal of Cell Biology, also reports new immunotherapy strategies that reverse this reprogramming process in mouse models of breast cancer metastasis.
“Metastatic disease is the main driver of breast cancer deaths, and we need a deeper understanding of how and why it occurs,” says Chan. “Our research has identified a new strategy for cancer cells to co-opt the immune system. If we could prevent or reverse natural killer cell reprogramming in patients, it could be a new way to stop metastasis and reduce breast cancer mortality.”
“Our study showed that NK cells selectively target the cells that initiate the metastatic process and also how the cancer cells trick the immune system into helping them,” says Ewald, senior study author, co-director of the Cancer Invasion and Metastasis Program at the Johns Hopkins Kimmel Cancer Center and professor of cell biology at the Johns Hopkins University School of Medicine. “This study also highlights the power of multidisciplinary cancer research. This project brought together medical oncology, cell biology, immunology and biomedical engineering to understand metastasis. We were able to move rapidly into immunology and immunotherapy research because of an exciting collaboration with Elizabeth Jaffee.”
Elizabeth Jaffee, M.D., is deputy director of the Johns Hopkins Kimmel Cancer Center; co-director of the Skip Viragh Center for Pancreas Cancer Clinical Research and Patient Care; associate director of the Bloomberg~Kimmel Institute for Cancer Immunotherapy; and the Dana and Albert “Cubby” Broccoli Professor of Oncology.
Using molecular profiling and computational analyses developed by Joel Bader, Ph.D., professor of biomedical engineering at the Johns Hopkins Institute for Basic Biomedical Sciences and Institute of Genetic Medicine, and Hildur Knútsdóttir, a fellow in Bader’s lab, Ewald said, they were able to map every suspected molecular interaction between immune cells and cancer cells — and identify the ones that were likely regulating this communication.
“As predicted, when we blocked these inhibitory signals, the NK cells continued to be the "good guys" and kept clearing out cancer cells,” Ewald says. “We’re excited this approach could be used to prevent metastases from forming, and we’re also testing whether this same approach could be used to reactivate an immune response to an existing metastasis.”
The investigators say the process may also apply to other cancer types. Immunotherapies that target NK cells could also potentially be used together with existing immunotherapies that stimulate T cells to fight cancer.
In addition to Ewald, Chan, Bader and Knútsdóttir, other researchers involved in this study include Veena Padmanaban, Manisha Warrier, Juan Carlos Ramirez, Matthew Dunworth, Hao Zhang and Elizabeth Jaffee.
The work was supported by the Breast Cancer Research Foundation/Pink Agenda; Twisted Pink; Hope Scarves; the National Cancer Institute (grants U01CA217846, U54CA2101732; 3P30CA006973 and T32CA009071); the Breast Cancer Research Foundation/Conquer Cancer Young Investigator Award; the Jayne Koskinas Ted Giovanis Foundation for Health Policy; and the Breast Cancer Research Foundation.
No authors declared conflicts of interest related to this research under Johns Hopkins University School of Medicine policies.
hopkinsmedicine.org


