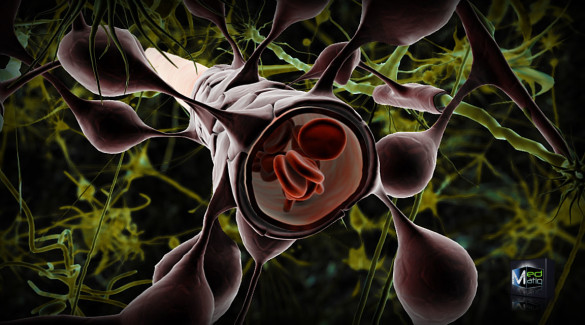
Illustration shows how the cells that make up the blood-brain barrier surround the blood vessels that run through the brain. This is the living structure that the new microfluidic device developed at Vanderbilt has succeeded in mimicking. (Ben Brahim Mohammed/Creative Commons License)
An interdisciplinary team of researchers from the Vanderbilt Institute for Integrative Biosystems Research and Education (VIIBRE) headed by Gordon A. Cain University Professor John Wikswo report that they have developed a microfluidic device that overcomes the limitations of previous models of this key system and have used it to study brain inflammation, dubbed the «silent killer» because it doesn’t cause pain but contributes to neurodegenerative conditions such as Alzheimer’s and Parkinson’s diseases. Recent research also suggests that it may underlie a wider range of problems from impaired cognition to depression and even schizophrenia.
The project is part of a $70 million «Tissue Chip for Drug Testing Program» funded by the National Institutes of Health’s National Center for Advancing Translational Sciences. Its purpose is to develop human organ-
The importance of understanding how the
Despite its importance, scientists have had considerable difficulty creating faithful laboratory models of the complex biological system that protects the brain. Previous models have either been static and so have not reproduced critical blood flow effects or they have not supported all the cell types found in human
Creating a blood-brain barrier on a chip
The new device, which the researchers call a NeuroVascular Unit (NVU) on a chip, overcomes these problems. It consists of a small cavity that is
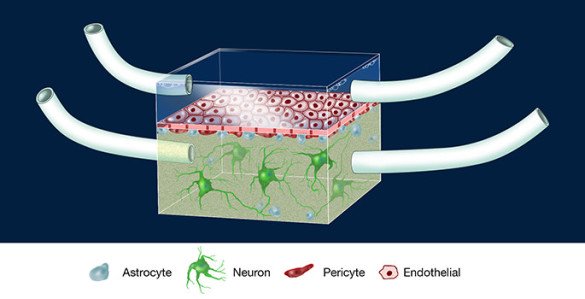
To create an artificial
After a day or two, when the endothelial cells have attached themselves to the membrane, the researchers flip the device and inject the two other human cell types that form the barrier —
The researchers were able to purchase the human endothelial cells, astrocytes and pericytes that they need from commercial sources. For the excitatory neurons required, they turned to Vanderbilt University Medical Center collaborators M. Diana Neely, research associate professor of pediatrics, and Aaron Bowman, associate professor of pediatrics, neurology and biochemistry. Starting with human induced pluripotent stem cells that are generated directly from adult cells they were able to produce the specialized neurons that the project needed.
«This is one of the most exciting projects I’m involved with," said Neely. «Although it’s still in its infancy, it has tremendous potential.»
According to Bowman, one potential application is to develop tissue chips that contain cells from individual patients, making it possible to predict their personal reactions to different drugs.
Device passes tests with flying colors
«Once we had successfully created the artificial barrier, we subjected it to a series of basic tests and it passed them all with flying colors. This gives us the confidence to state that we have developed a fully functional model of the human
«The NVU has reached the point where we can begin using it to test different drugs and compounds," observed team member Donna Webb, associate professor of biological sciences who is interested in studying how different substances affect synapses — the junctions between neurons. «There is an urgent need for us to understand how various substances affect cognitive processes. When we do, we will be in for a number of surprises!
Providing the first continuous picture of inflammation response
Already, the VIBRE team has used the NVU to overcome a basic limitation of existing studies of brain inflammation, which have only produced snapshots of the process at various stages. Because the NVU can be continuously monitored, it has provided the first dynamic view of how the brain and
These results are summarized in a paper titled «Metabolic consequences of inflammatory disruption of the
The scientists exposed the NVU to two different compounds known to induce brain inflammation: a large molecule found on the surface of certain bacteria called lipopolysaccharide and a «cocktail» of small proteins called cytokines that play an important role in immune response to inflammation.
«One of our biggest surprises was the discovery that a critical component in the
The researchers also found that the blood vessels in the barrier respond to inflammation by pumping up their metabolic rate while the metabolism of the brain cells slows down. According to Brown, «It might be that the vasculature is trying to respond while the brain is trying to protect itself.»
Commercialization potential
The technology underlying the NVU has been patented and is available for commercialization. Interested parties should contact Ashok Choudhury or Masood Machingal at the Vanderbilt Center for Technology Transfer and Commercialization.
Funding for the research was provided by National Institute of Environmental Health Sciences award R1 ES016931, National Institutes of Health grant DK50435 and National Center for Advancing Translational Sciences award


