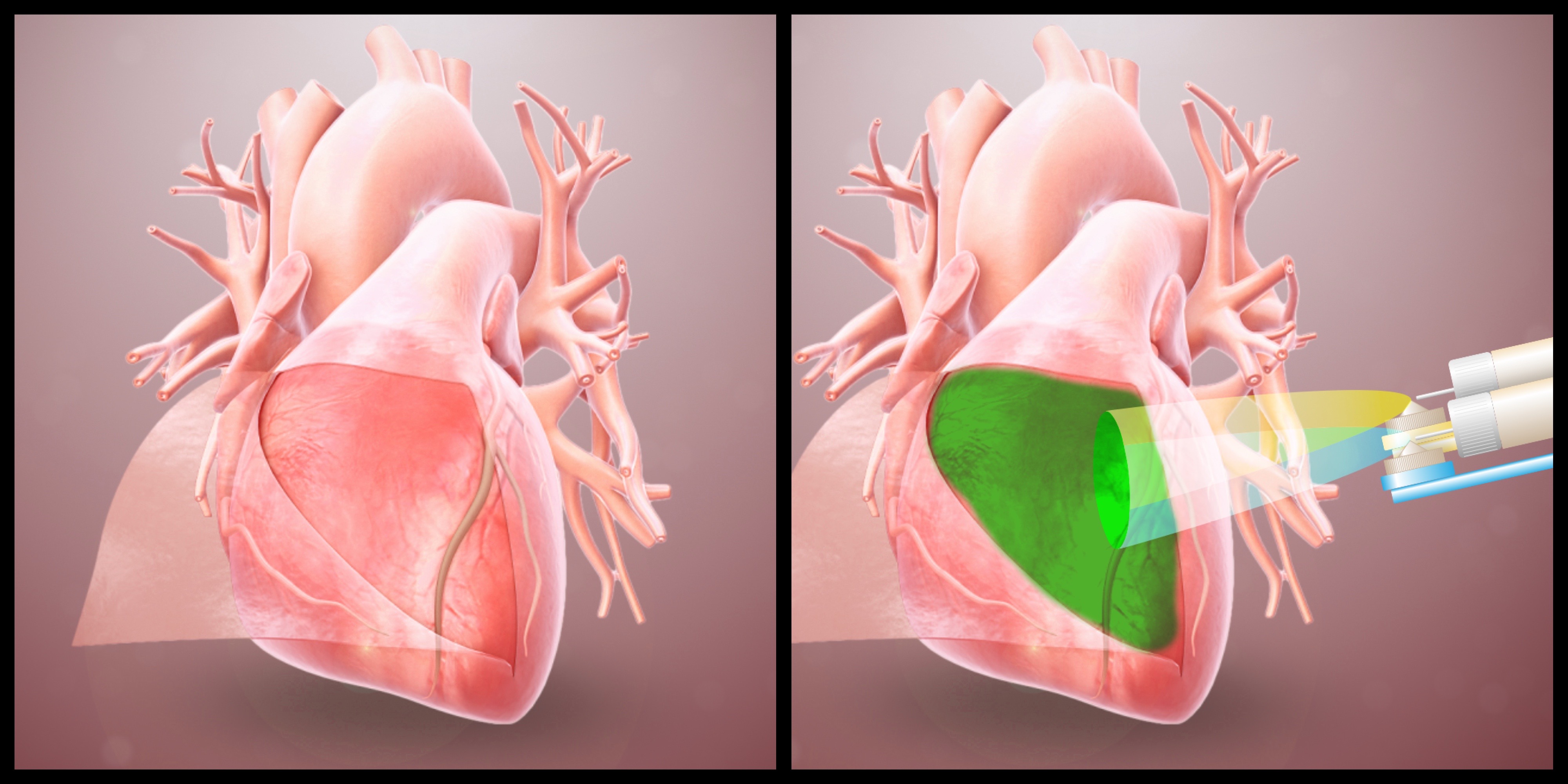
 Researchers also developed a device to spray the hydrogel onto the heart.
Researchers also developed a device to spray the hydrogel onto the heart.Adhesions—organ tissue sticking to surrounding tissue—are a relatively common problem when surgeons need to operate again at the same site, which happens in 20 percent of cases every year in cardiac surgery. Re-operations are particularly common when the patients are children suffering from cardiac malformations—as the child’s heart grows, additional interventions are needed.
Adhesions form within the first 30 days post-op and can complicate operations and increase the risk of mortality during interventions. In some cases, they can also interfere with proper heart function or completely prevent a repeat surgery. One of the paper’s senior authors, UC San Diego bioengineering professor Karen Christman, experienced this when one of her uncles couldn’t have a heart valve repaired because of severe adhesions.
“Our work is an engineering solution driven by a medical problem,” said Christman, who co-founded a company, Karios Technologies, to bring the hydrogel into the clinic. “And now it’s poised to significantly improve cardiac surgery, both for adults and children.”
The work brought together not only bioengineers and physicians, but also chemists and materials scientists.
In academic medical centers such as UC San Diego, most surgeons conduct repeat operations and encounter adhesions fairly regularly. In this study, in rats, 70 percent of animals in the control group developed severe adhesions.
Currently there are no FDA-approved products marketed for preventing adhesions after heart surgery. “This approach has the potential to significantly impact the lives of many patients who may require repeat operations, either on the heart or elsewhere in the body,” said Dr. Michael M. Madani, chair of the Division of Cardiovascular and Thoracic Surgery at UC San Diego Health and one of the paper’s co-authors.
How it’s made
By contrast, the hydrogel developed by bioengineers in Christman’s lab is designed specifically to meet both the patients’ and surgeons’ needs. It’s sprayable, so easy to apply. Once sprayed onto tissue, it binds to the heart muscle and turns into a soft, elastic coating that creates a protective barrier, while still allowing for movement. The gel can be easily removed from tissue and dissolves after more than four to six weeks.The biggest challenge was making sure that the hydrogel attaches strongly enough to the heart but doesn’t swell, as swelling can put dangerous pressure on the heart. Christman and team used what’s known as crosslinking chemistry, which consists of linking two molecules together with a covalent bond, to accomplish this. Masaki Fujita, the paper’s first author and a visiting scientist in the Department of Bioengineering at UC San Diego, had the idea of using a compound known as catechol, similar to what mussels use to adhere to rocks, to ensure the hydrogel stayed in place on the heart.
Catechol contains an amino acid, L-dopa, that is a muscle binding protein. In this case, it was added to the gel base, a water soluble polymer, known as PEG. The result is a hydrogel that sticks onto the organ it is applied to, but then creates a protective barrier that lasts at least up to four weeks before dissolving. By that point, adhesions are less likely to form. To the researchers’ knowledge, it’s the first time this type of formulation has been used for preventing adhesions after surgery.
Spraying device
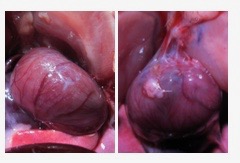 Left: a rodent heart treated with the hydrogel post-op shows little to no adhesions two weeks post-op. Right: a heart from the control rodent group shows severe adhesions.
Left: a rodent heart treated with the hydrogel post-op shows little to no adhesions two weeks post-op. Right: a heart from the control rodent group shows severe adhesions.Researchers also designed a device to safely and accurately spray the hydrogel inside the area where open heart surgery is being performed. The device houses the hydrogel’s two main components in two different chambers. Each component is made of PEG with different reactive groups that crosslink together to form the hydrogel. One of the solutions also includes the catechol-modified PEG to ensure it stays on the heart. The two mix as they exit the device, forming a gel. The process is akin to using two cans of spray paint, for example blue and yellow, to create a third color, green.
Next steps and bigger picture
The next step is to do a large-scale trial in pigs to refine dosage and examine how the hydrogel binds to sutures and drains. The ultimate goal is to conduct a human pediatric study in 18 months to two years and bring the product to the FDA for approval in five years.Karios Technologies is licensing the technology from UC San Diego. “We want feedback from surgeons,” Gregory, CEO of Karios Technologies said. “We designed this material specifically for use on the heart and ease of use by the surgeon.”
The technology could easily translate to other organs also requiring multiple operations and susceptible to adhesions, researchers said.
The work was funded by the National Heart, Lung and Blood Institute through the UC Center for Accelerated Innovation, and the National Center for Advancing Translational Sciences through the UC San Diego Center for Clinical and Translational Research Institute.
Christman co-founded and holds equity interest in Karios Technologies, a startup that aims to commercialize the hydrogel technology. Dr. Madani is a consultant for the company. Christman, Fujita and Madani are inventors on patents and patent applications related to the work.
MEDIA CONTACT
Ioana Patringenaru
ucsdnews.ucsd.edu


