On a global scale, the condition affects three to eight percent of pregnant women, and in 2013 preeclampsia resulted in 29,000 deaths. Unfortunately, and because of the ethical difficulty in testing pregnant women, little is known about the potentially fatal condition, and the only route for treatment is usually a premature delivery. Recently, however, the doors for preeclampsia research have opened thanks to scientists at the Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National Health System and researchers from the University of Maryland who successfully 3D bioprinted a placenta model.
The study about the 3D bioprinted placenta model, which was recently published in American Chemical Society (ACS) Biomaterials Science & Engineering, could be the key to discovering the cause of preeclampsia as its cellular composition and functions mimic the real organ. By observing and testing the additively manufactured placenta, made by the layering of living cells, the scientists have allowed for «unprecedented opportunities» in understanding and treating placenta related disorders such as preeclampsia.
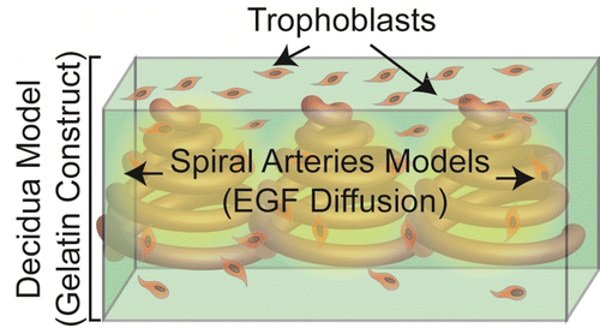
According to the study published by the researchers, they have been able to use the 3D bioprinted placenta model to observe and document the migration of trophoblasts (nutrient cells in the placenta), which some believe, if abnormal, could be the cause of preeclampsia. As John P. Fisher, chair of the Fischell Department of Bioengineering, University of Maryland explains in a press release, «Our study provides a proof of concept that a 3D bioprinted placenta model is a viable way to study and understand the dynamics of cell migration in the formation of the placenta. What we have learned from this initial study is a significant step toward understanding the cause of preeclampsia and a potential therapy.»
With the bioprinted model’s key cellular, biochemical, and extra cellular matrix components, the scientists have been able to recreate and monitor trophoblast migration as well as the effect of epidermal growth factor (EGF) on the trophoblasts’ migration behavior. The study found that EGF, which stimulates cell growth, proliferation, and differentiation, had a positive effect on the trophoblasts in the 3D bioprinted placenta, which could in turn lead to a potential treatment for preeclampsia.
«A 3D model provides you with a much clearer picture of cell behavior," explains
Dr. Peter C. Kim, vice president and associate surgeon in chief, Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National Health System, adds, «Until now, there has been a lack of effective experimental placenta models. Animal models are not directly relevant and are misleading as the placentation process in humans is very different from those of other species. At the same time, clinical testing involving pregnant mothers is not feasible due to ethical and regulatory considerations, so a new solution was needed.»
With the successful 3D bioprinting of the placenta model, the team of scientists are on the right track towards developing even more advanced bioengineered placenta models, which will continue to help and advance towards treatments for placenta related conditions such as preeclampsia, as well as placenta accreta and placenta previa. The significance of the study is exceptional as the 3D bioprinted placenta models could help to save many mothers and infants down the line.
The Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National Health System was founded in 2010 through a $150 million donation from the government of Abu Dhabi. The institute, which conducts research to advance pediatric surgery, is based at the Children’s National Health System in Washington, DC, which itself has been treating children since 1870.
Source: http://www.3ders.org/articles/20160519-3d-bioprinted-placenta-model-could-be-the-key-to-treating-pre...


