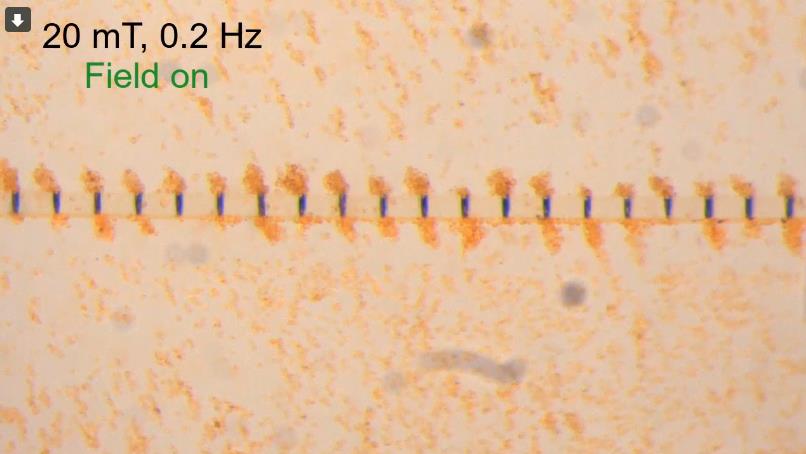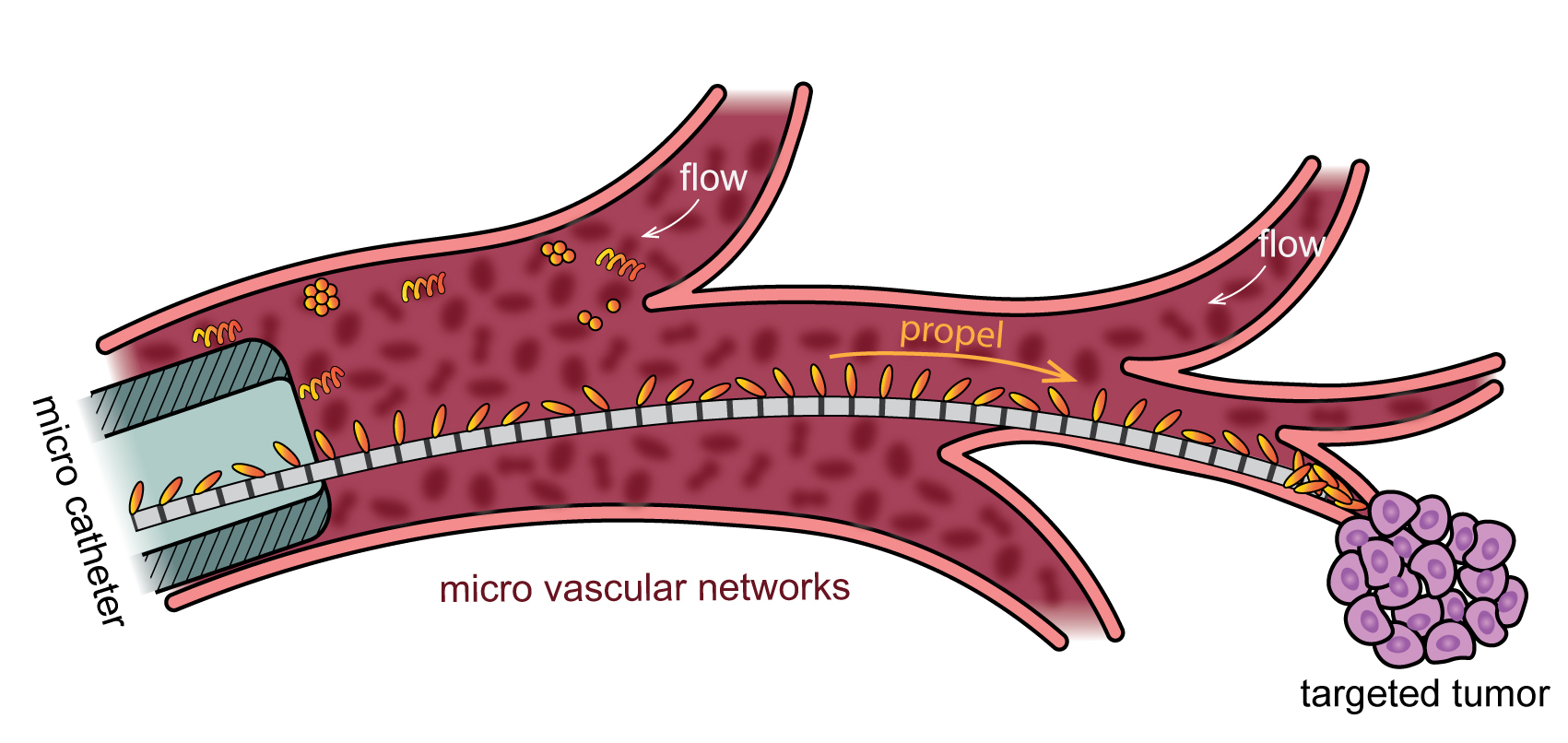
While free-swimming microrobots have been explored as a way to precisely deliver therapeutics within a blood vessel, they can disperse in the strong flows, failing to reach their target at high enough concentrations. In contrast, microrobots propelled along an artificial microtubule, developed by physicist Arnold Mathijssen and colleagues, can be transported precisely, even working against the current. (Image: Courtesy of Arnold Mathijssen/Nature Machine Intelligence)
Like a microscopic bucket brigrade, an artificial microtubule can rapidly transport tiny particles along magnetic stepping stones, delivering them to a precise location even when operating against a strong current.
The technology, developed by a team from the University of Pennsylvania and ETH Zürich, may one day facilitate the delivery of targeted therapies through the bloodstream to treat blocked vessels or cancerous tumors.
The findings are published in the journal Nature Machine Intelligence.
Researchers have explored the potential of microrobots to “swim” in the bloodstream as a way of directing drugs to the exact location where they are needed. The drawback of this approach is that freely swimming microrobots struggle to make headway against the complex fluid flows that exist inside the human body.
“As a result, you often see dispersion of the particles that you would like to deliver,” says Arnold Mathijssen, a corresponding author on the work and an assistant professor in Penn’s Department of Physics & Astronomy. “Really what you would like to achieve is to have the largest concentration of the therapeutic at one site and not have it disperse anywhere else, as that could result in toxicity.”
Catheters and microneedles have until now been the techniques of choice to complete these directed interventions. Yet catheters can only be miniaturized so far before they lack the pumping force necessary to transport microscopic cargo. Similarly, even microneedles are still too large to reach the narrowest blood vessels.
To overcome these obstacles, Mathijssen and colleagues looked to biology for inspiration.
“When you look in nature, inside cells there is a beautiful solution,” Mathijssen says. “Microtubules, which are part of the cytoskeleton, use molecular motors to transport vesicles to different locations in the cell. These motors find a way to deal with the fluctuations in flow that we see in blood vessels and elsewhere in the body. We wanted to try to synthesize something similar in a nanotechnology setting to see if we could use it as an efficient delivery mechanism.”
Their bio-inspired design was an artificial microtubule, fabricated first in Switzerland and later at Penn’s Singh Center for Nanotechnology. These thin fibers, composed of crosslinked polymers to give them elasticity, were embedded with magnetic plates made of nickel, interspersed at defined distances like stepping stones. Just 80 microns in width, the microtubules would be narrow enough to slip through narrow blood vessels.
Applying a rotating magnetic field around the artificial microtubules turns the nickel stepping stones into magnets, along which a cargo of metal microrobots “walk,” one to the next.

“We place the microtubules in a rotating magnetic field, just like an MRI machine,” Mathijssen says. “If you rotate the field slowly, the particles move slowly, and when you rotate faster the particles speed up as well.”
There was a “sweet spot” in the magnetic field strength, the scientists found; rotating too fast caused the particles to slip on the surface and disperse away from the microtubule.
In experiments testing the transport mechanism’s performance in blood vessel-like networks, the research team found that the microparticles could travel along the microtubule fiber even when subjected to strong fluid flows, tuned to replicate the dynamicism of blood flow. Compared to existing technologies, the microcargo delivery proceeded rapidly, an order of magnitude faster. And fine adjustments to the magnetic field ensured the cargo could be delivered accurately to the intended location, even in complex vessel networks.
Not only does this new innovation draw from nature, but Mathijssen notes it can in turn give insights into how biological systems operate. He and his colleagues observed that, when the microparticles moved between stepping stones, they would self-assemble, forming clumps, each tethered to one of the stepping stones. Eventually the assembled particles would push each other forward in a collective effort. While a few other groups have suggested that this might occur inside cells to enhance cytoskeletal transport, this work provides the first experimental evidence of propulsion principle.
“Sometimes you build something in the lab and it can tell you something new about biology,” he says.
To apply this microparticle transport strategy in the real word, the researchers are envisioning swapping out nickel, which is toxic, for other materials, such as iron oxide, which is already FDA-approved for internal use. They’re also keeping an open mind as to the ways the microtubules could be used. Targeted drug delivery and blood vessel plaque removal are obvious applications, but Mathijssen is also imagining the benefits of a two-dimensional fiber. Wrapped around medical devices. Such a device could deliver antimicrobials to prevent the growth of dangerous bacterial biofilms.
“We believe these ‘microhighways for microrobots’ can provide an altenative solution to free-swimming microrobots and other current technologies,” he says, “bringing robust biomedical microtransport much closer to reality.”
Arnold Mathijssen is is an assistant professor in the Department of Physics & Astronomy in the School of Arts & Sciences at the University of Pennsylvania.
Mathijssen coauthored the study with ETH Zürich’s Hongri Gu, Emre Hanedan, Quentin Boehler, Tian-Yun Huang, and Bradley J. Nelson. Mathijssen, Gu, and Nelson were co-corresponding authors.
The study was supported by the European Research Council (Grant 743217), the Swiss National Science Foundation (Grant 200020B_185039), and ETH (Grant 1916-1).
Writer: Katherine Unger Baillie